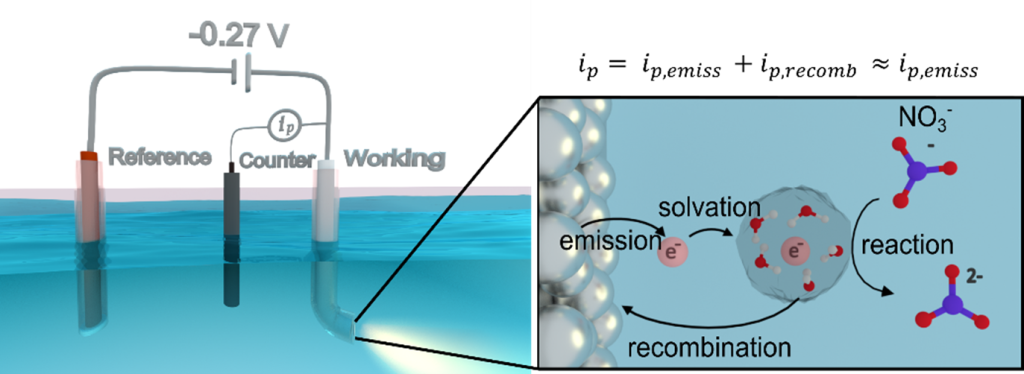
Energetic carriers can be generated from plasmon decay at the metal interface. However, effectively harnessing these hot carriers presents a significant challenge. When a plasmonic nanoparticles interfaces with a liquid solution, hot electrons can be transferred into the solution and stabilized by surrounding solvent molecules, forming solvated electrons. These solvated electrons possess strong reducing potential, making them promising drivers for various redox chemistries in a homogeneous environment. While their non-plasmonic generation typically requires harsh physical and chemical conditions that can also create other reactive species, limiting their applicability, plasmon-induced solvated electron production occurs selectively under comparatively mild light excitation.
In the Link Research Group, we are investigating the mechanisms of plasmon-induced solvated electron generation and are pioneering ways for how to increase solvated electron yields. Our goal is to achieve high quantum efficiencies through the strong near-field of optimally designed plasmonic nanoelectrodes under mild electrochemical conditions and low-powered continuous-wave light.
Ultimately, we aim to leverage plasmon-enhanced photoemission to conduct controlled redox chemistry with solvated electrons, opening new pathways for selective synthesis of valuable chemicals, production of fuel molecules, or degradation of environmental hazards.
Selected publication:
- A. Al-Zubeidi, B. Ostovar, C. C. Carlin, B. C. Li, S. A. Lee, W,-Y, Chiang, N. Gross, S. Dutta, A. Misiura, E. K. Searles, A. Chakraborty, S. T. Roberts, J. A. Dionne, P. J. Rossky, C. F. Landes, and S. Link, Mechanism for plasmon-generated solvated electrons, PNAS, 120, 3, e2217035120 (2023)